Abstract
Introduction
The prospective randomized study on treatment of 192 severe aplastic anemia (SAA) patients with ATG and Cyclosporine (CSA) with and without G-CSF showed that G-CSF added to ATG/CSA decreases the rate of early infection episodes and days of hospitalization in very SAA (vSAA) patients, but has no significant impact on overall survival (OS), event free survival (EFS), relapse, or death rates (Blood, 2011;17:4434). The number of secondary MDS/AML was low, however with a short observation time. Now, 16 years after initiation of the study, a follow-up was planned to evaluate long-term outcome, comparing patients with and without G-CSF.
Patients and Methods
A total of 192 patients with newly diagnosed SAA, not eligible for stem cell transplantation (SCT) were entered into this prospective randomized multi-center study to receive ATG/CSA with (49.5%) or without G-CSF (50.5%). In 2011, 44 of the 192 patients had died. For the present study the follow-up of the 148 patients alive at time of first publication were requested. There were 49% males (49% G-CSF; 48% no G-CSF), 36% with vSAA (32% G-CSF; 41% no G-CSF). The median age at randomization was 46 years (2-80), 47 (2-80) for the G-CSF and 44 (7-80) for the non-G-CSF group. The median follow-up using reverse KM method was 11.7 years (10.9-12.5).
Results
Among the 110 survivors (17 missing), 71 (65%) were in CR, 33 (30%) in PR and 6 not in remission (5%), without any difference between the G-CSF and non-G-CSF group (P=0.523). At last follow-up 65 (34%) of the patients have died. Causes of death were infection (26), bleeding (3), SAA unspecified (3), MDS/AML (4), solid cancer (3), transplant related mortality (8), cardiovascular/aging (7), or unspecified (11). There was no difference in the causes of death between patients treated with or without G-CSF.
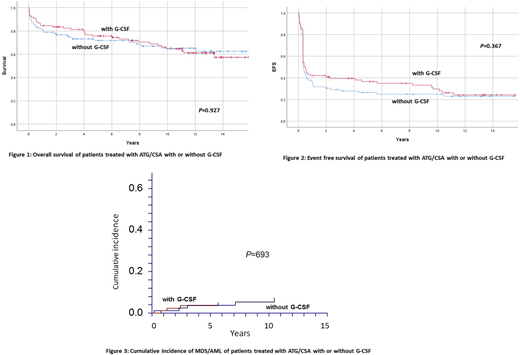
OS at 15 years was 57±12% for the G-CSF and 63±12% for the non-G-CSF group (P=0.927). EFS, including SCT, relapse, non-response at day 120, second MDS/AML, PNH or death as an event, was 24±10% for the G-CSF, and 23±10% for the non-G-CSF group (P=0.367). Nine patients developed florid or morphological signs of MDS/AML, 9 clonal cytogenetic anomaly only, 7 a solid cancer, 18 clinical PNH, 8 avascular osteonecrosis, and 12 chronic kidney disease (No difference between patients treated with or without G-CSF).
Cumulative incidence (CI) at 15 years of MDS/AML (isolated cytogenetic anomalies not included) was 5.0±2% (G-CSF) and 7.3±3% (no G-CSF), respectively (P=0.693); for clinical PNH it was 10.1±5% and 13.3±7% (P=0.499), for relapse of responding patients at day 120, 29.8±22% and 25.1±17% (P=0.545), and for chronic kidney failure 16%±12% and 13%±12% (P=0.513), respectively. Forty patients needed a second line immunosuppressive therapy (IST) for relapse (17), refractory disease (8), cyclosporine dependence (6) or isolated cytopenia (9) (G-CSF 26; no G-CSF 16; P=0.291); 16 patients needed a third line IST for relapse (5), refractory disease (7), isolated cytopenia (4) (G-CSF 12; no G-CSF 4; P=0.647). Twenty-eight patients were treated with allogeneic SCT in second or subsequent line (G-CSF 12; no G-CSF 16). CI at 15 years of SCT (competing risk, death without SCT) was 14±8% (G-CSF) and 22%±10% (no G-CSF), respectively (P=0.380). OS at 10 years since SCT was 46±24%.
The most important risk factors for patients treated with ATG/CSA with or without G-CSF were age and severity of the disease at randomization: OS at 15 years was 89±12% (<20 years), 81±13% (20-40 years), 55±15% (40-60 years), and 32±16% (>60 years of age), respectively (P>0.001), and 64±5% and 52±7% for patients with SAA and vSAA, respectively (P=0.021). There was no difference between patients treated with or without G-CSF. Finally, the lack of neutrophil response by day 30, which was significant at first evaluation, is still associated with borderline lower survival (46.6±14% versus 67.1±9%; P=0.058).
Conclusion:
Long-term outcome of SAA patients treated with ATG/CSA was not influenced by supplementing G-CSF in term of OS, EFS, death rates, relapse, PNH, secondary MDS/AML, solid cancer and non-malignant late complications. Due to the pre-cancer nature of the disease and its long-lasting treatment, patients treated with IST are at risk for a number of malignant and non-malignant late complications. It is somewhat disappointing that in this careful followed cohort less than 25% of patients are alive and event-free 10-15 years after initial treatment.
Peffault De Latour:Pfizer Inc.: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Hoechsmann:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding. Schrezenmeier:Alexion Pharmaceuticals, Inc.: Honoraria, Research Funding. Kulasekararaj:Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support . Bader:Neovii: Research Funding; Cellgene: Consultancy; Riemser: Research Funding; Medac: Patents & Royalties, Research Funding; Novartis: Consultancy, Speakers Bureau. Risitano:Pfizer Inc.: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amyndas Pharmaceuticals: Consultancy; Alnylam Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Ra Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal